Hormones
Male pattern baldness is caused by elevated levels of dihydrotestosterone (DHT) hormone. Dihydrotestosterone is a metabolic product of the conversion of free testosterone by 5-alpha reductase. DHT causes the progressive shrinkage of hair follicles which may eventually lead to their becoming dormant.
Ageing
Due to changes in hormonal levels as a person ages, onset of baldness with age may also take place. Aging causes a progressive decrease in the Anagen hair (growth phase of hair cycle) and leads to an increased number of dormant hair follicles.
Genetics
Baldness is dependent on several genetic factors. It is strongly associated with the AR gene on the X chromosome. About 80% of the cases of Androgenetic Alopecia are due to genetic factors.
Metabolic Syndrome
Premature baldness may be caused by an interplay of several factors such as nutritional deficiencies, glucose uptake, insulin resistance, androgen levels and other genetic factors. These must be carefully investigated while evaluating male pattern baldness.
Minoxidil
Minoxidil is a medication used topically or orally for the treatment of hair loss. It is a potent vasodilator that acts by providing increased blood supply and thereby, more nutrition to the hair follicle.
Finasteride
Finasteride is a drug that is a DHT blocker, which prevents the binding of DHT to the hair follicle, thereby inhibiting hair follicle shrinkage and slowing the progression of male pattern baldness in patients. Main objective of this therapy is to decrease the rate of baldness in an individual.
Platelet Rich Plasma Treatment
PRP treatment involves the separation of platelet rich plasma from the patient’s own blood and its microinjection into the balding areas of the scalp. The growth factors and proteins present in the plasma then regenerate the shrinking hair follicles and result in improved hair growth.
Stem Cells And Exosomes
Stem cells are pluripotent cells that hold the potential to differentiate into stimulatory cells providing increased growth factors to the hair follicle.
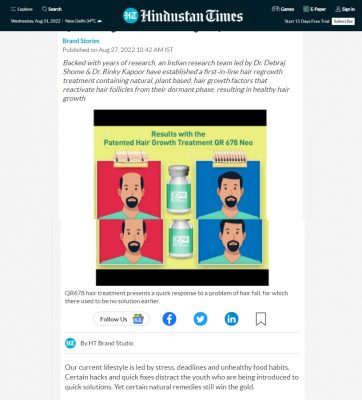
The QR678® Neo therapy in Androgenetic Alopecia:
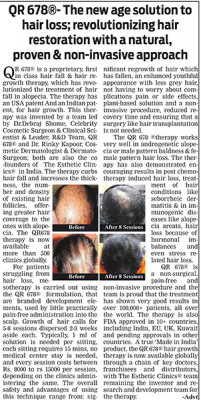
he QR678® Neo therapy is a proprietary, first in class hair fall & hair regrowth therapy, which has revolutionized the treatment of hair fall in alopecia. This formulation was invented in India & has been named QR678 inspired by the new generation ubiquitous presence of “Quick Response“ QR code. 678 in Morse Code signifies “there is no answer”. This formulation has been named QR678® to signify a “Quick Response to a disease which earlier had no answer”.
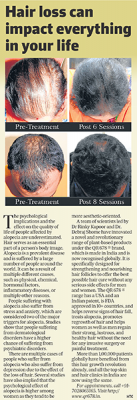
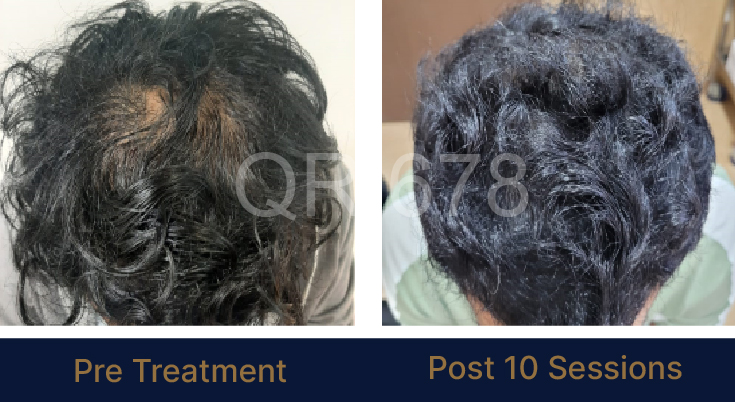
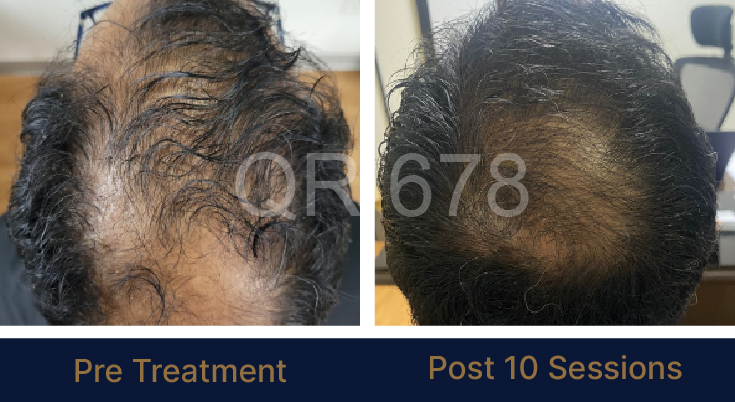
Before & After Results
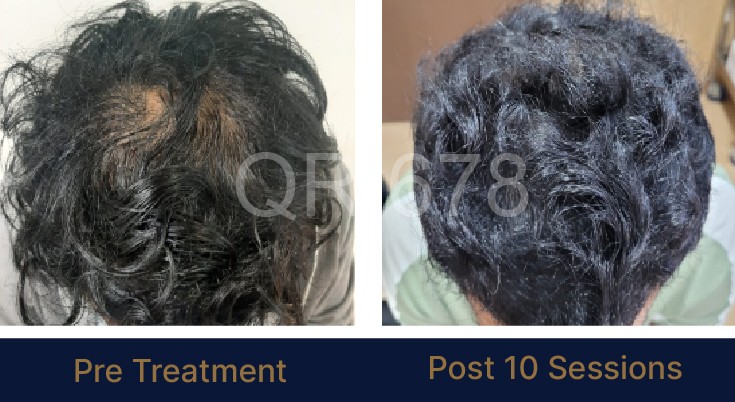
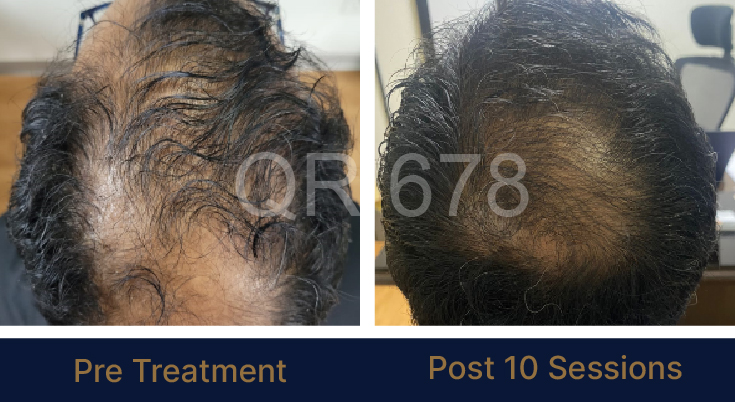
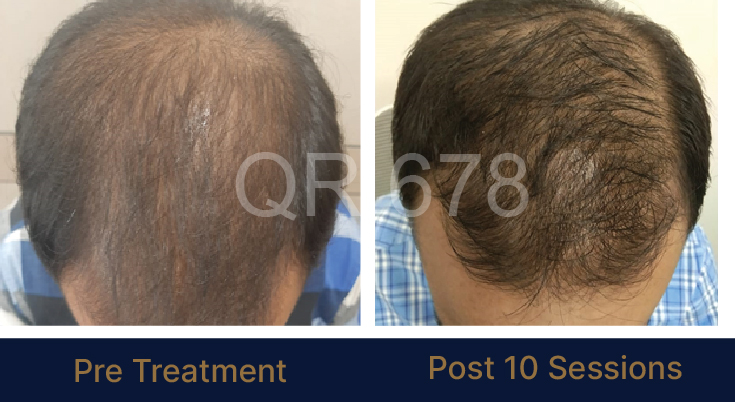
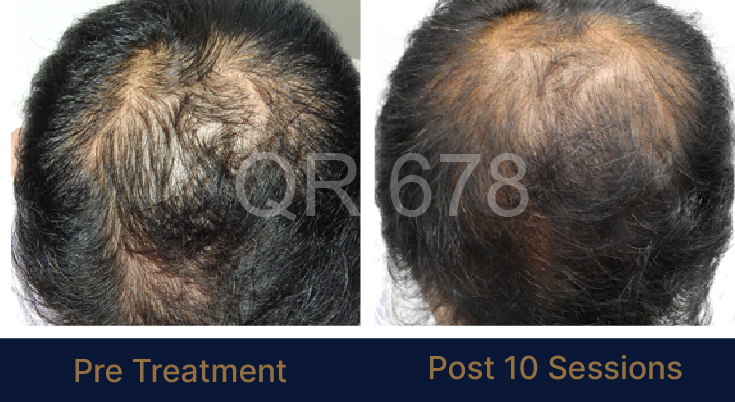
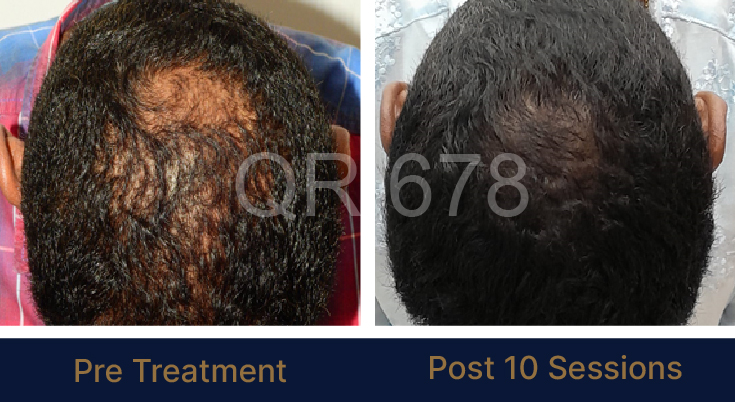
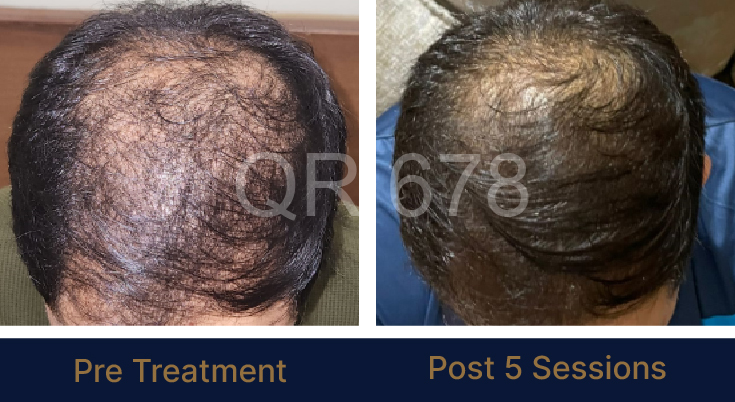
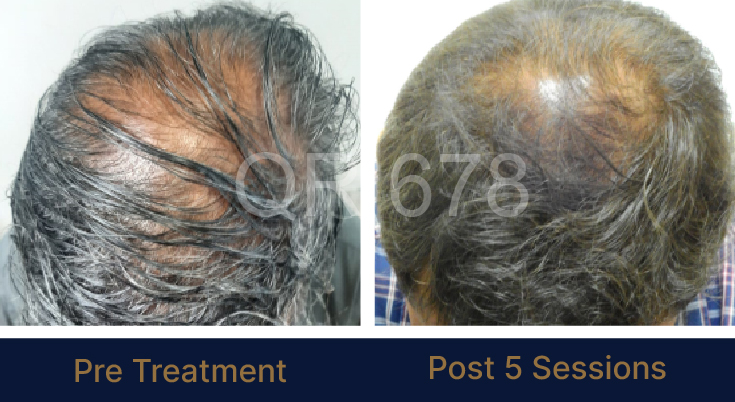
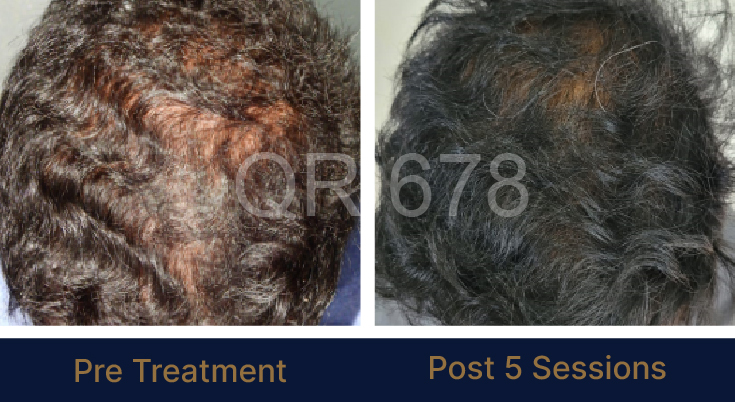
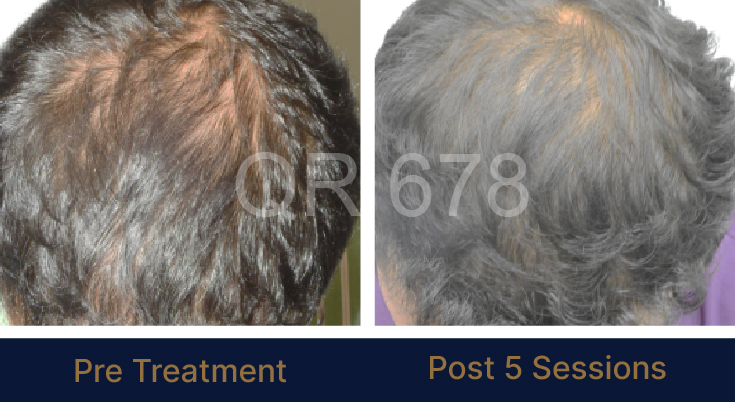
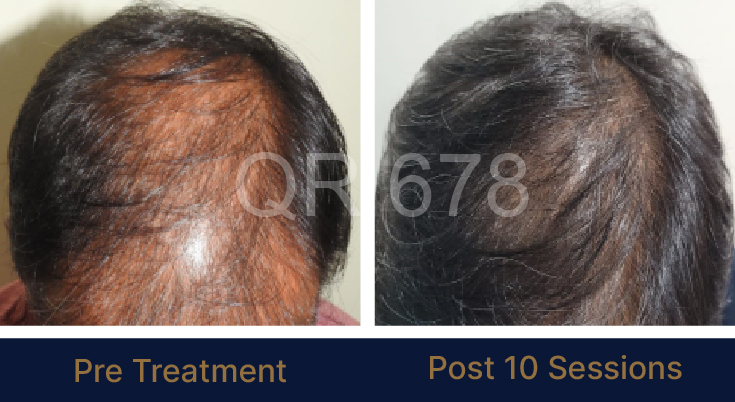
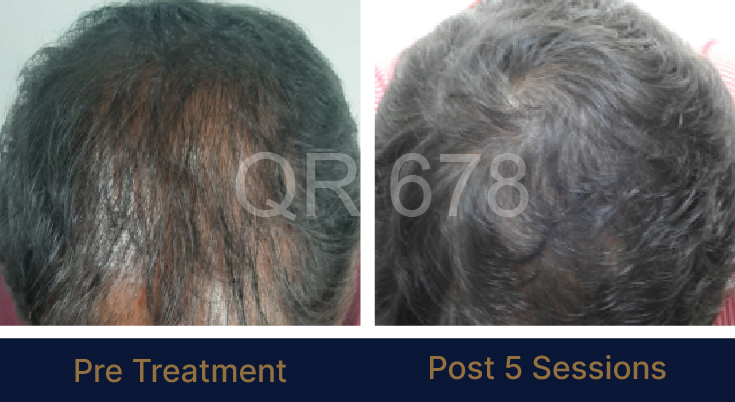
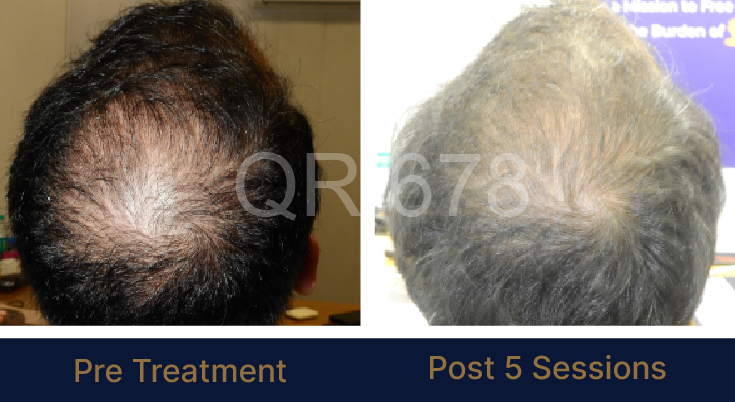
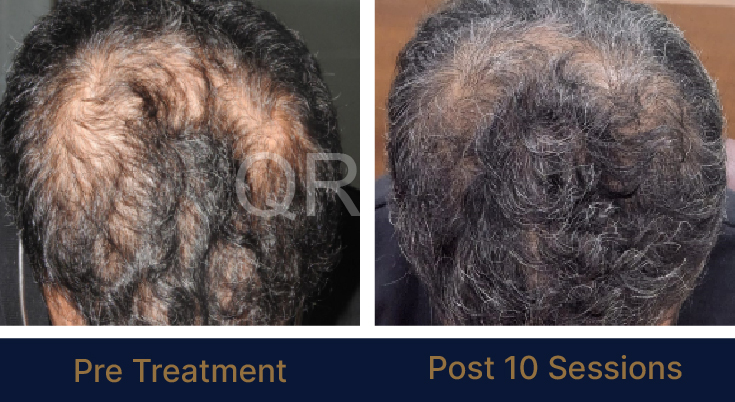
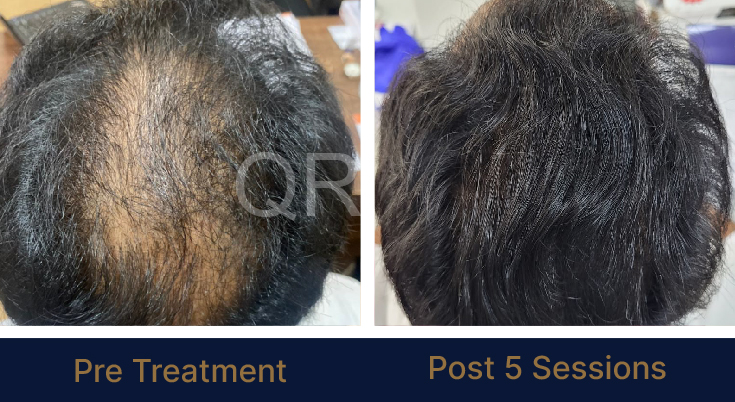
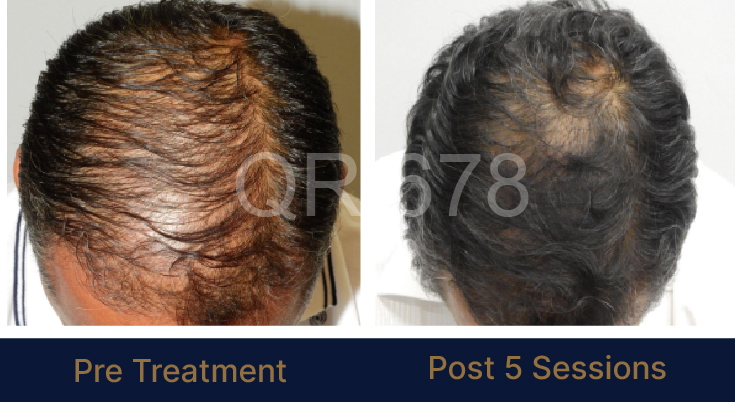
Before & After Results
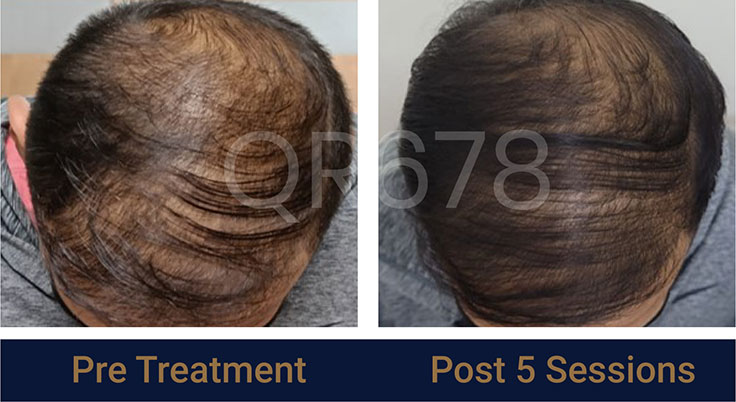
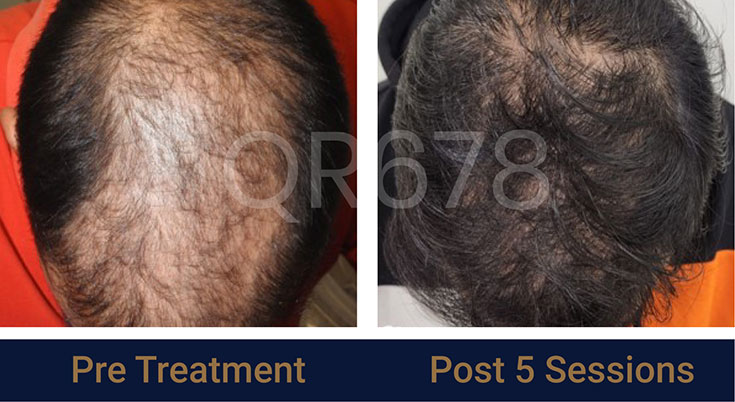
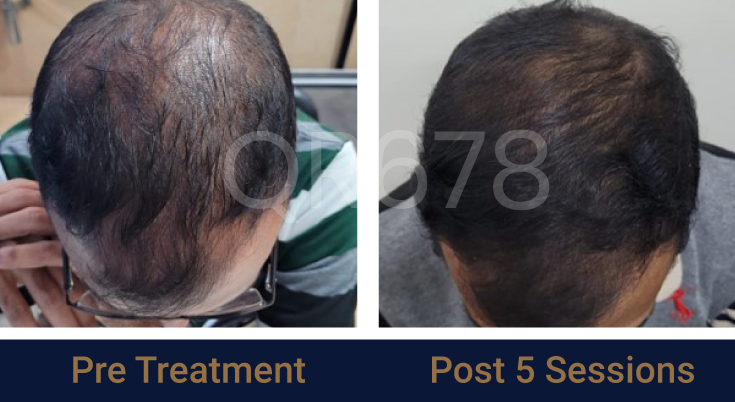
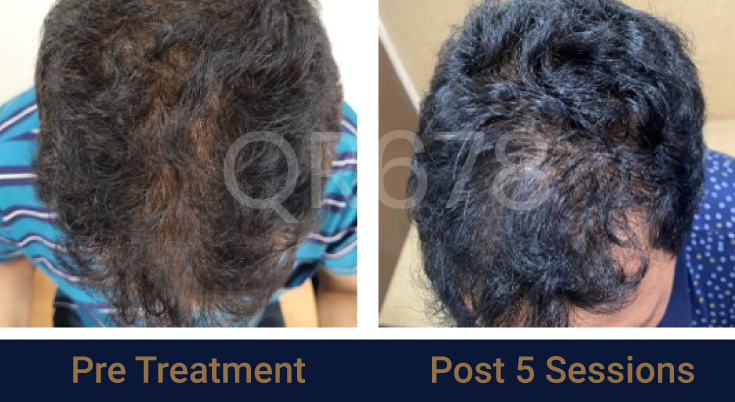
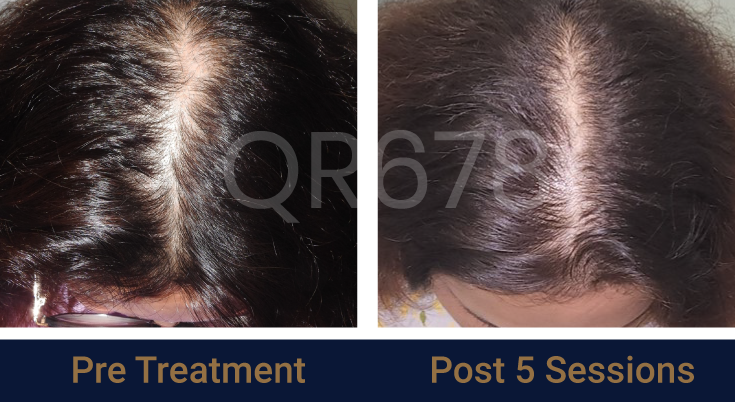
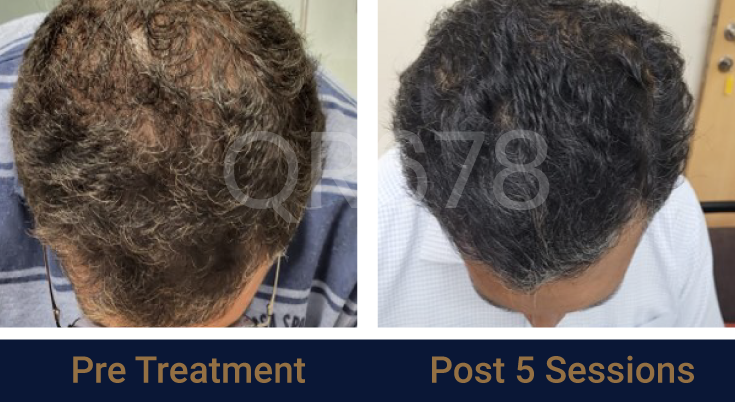
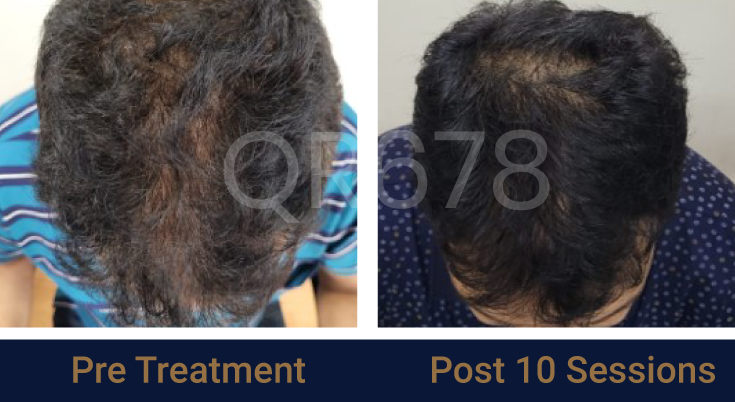
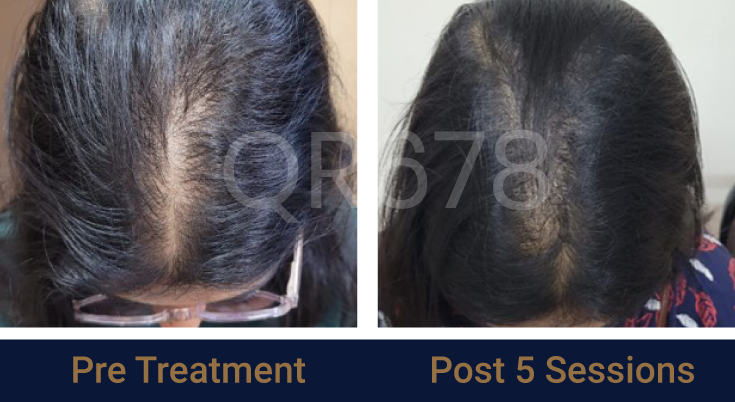
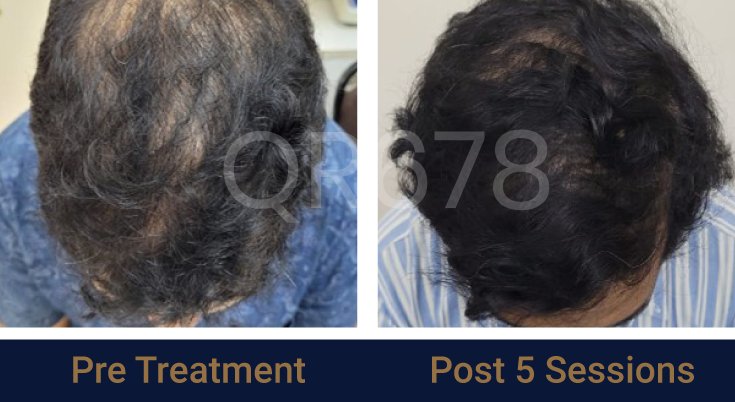
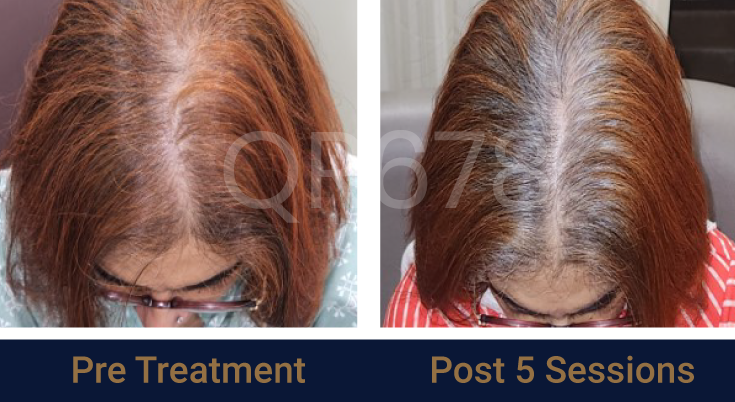
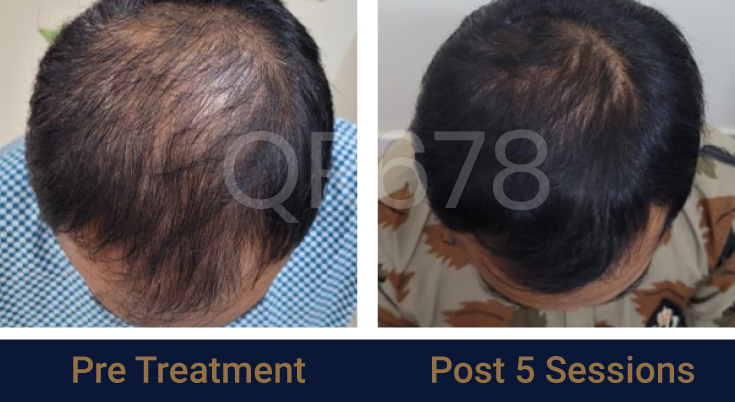
Effects of QR678® Neo in androgenetic alopecia in
comparison to topical
minoxidil:
Topical minoxidil and oral finasteride tablets.
The most important clinical trial evaluating the efficacy of minoxidil in androgenetic alopecia was performed in the USA – https://clinicaltrials.gov/ct2/show/NCT00151515
This clinical trial though was not neutral and was industry (Johnson & Johnson, manufacturer & patent holder for minoxidil) sponsored.
The primary purpose of the study was to evaluate the efficacy of a topical 5% minoxidil formulation in males for the treatment of male pattern hair loss. The secondary purpose was to evaluate the safety of a topical 5% minoxidil formulation in males when used twice daily for the treatment of pattern hair loss and to obtain the safety data on the investigational product when used twice daily for up to one year.
In August 1988, the US FDA finally approved minoxidil for treating baldness in men under the trade name “Rogaine”. The agency concluded that although “the product will not work for everyone”, 39% of the men studied had “moderate to dense hair growth on the crown of the head”. This medicine was then recommended for female pattern hair loss, as well.
Minoxidil is generally well tolerated, but common side effects include burning or irritation of the eye, itching, redness or irritation at the treated area, and unwanted hair growth elsewhere on the body. Dandruff is a common problem as is Temporary hair loss. Manufacturers note that minoxidil-induced hair loss is a common side effect and describe the process as “shedding”.
We believe that minoxidil works well to stop or reduce hair fall in a majority of people, but does not work as well at hair regrowth.
Regular usage of minoxidil leads to compliance issues & most people do not use minoxidil in the recommended twice a day usage pattern, used in clinical trials.
As the QR678® Neo has no real side effects & causes hair growth, especially in minoxidil resistant cases, it is an interesting alternative to minoxidil & is preferred by a large number of patients.
Effects of QR678® Neo in
female pattern alopecia in
comparison to oral
finasteride:
Finasteride, a type 2-selective 5α-reductase inhibitor, was approved in 1997 as the first oral pharmacologic therapy for the treatment of men with androgenetic alopecia (Male pattern hair loss).
Three double-blind, randomized, placebo-controlled Phase III studies of finasteride were conducted in a sample of 1879 men aged between 18–41 years with mild to moderate AGA. Two of the studies enrolled men with predominantly vertex hair loss and one study enrolled men with predominantly frontal (anterior mid-scalp) hair loss. Finasteride 1 mg or matching placebo tablets were taken once daily for 24 months in the vertex studies and for 12 months in the frontal study. All three studies demonstrated that finasteride treatment led to a significant improvement in hair growth and slowing of further hair loss progression, while placebo-treated men showed significant hair loss.
The net improvement in hair count (finasteride vs. placebo) was 14% at 1 year and 16% at 2 years. Only 17% of men receiving finasteride lost hair based on hair count during the 2-year studies, compared with 72% of men receiving placebo. These data demonstrated that finasteride slowed the progression of hair loss.
The problem though was that drug-related adverse events reported by ≥1% of men receiving finasteride 1 mg in the first year were decreased libido (reported by 1.8% of men on finasteride vs. 1.3% on placebo), erectile dysfunction (reported by 1.3% of men on finasteride vs. 0.7% on placebo) and ejaculation disorder (1.2% of men on finasteride vs. 0.7% on placebo). Approximately 1% (1.2%) of men receiving finasteride discontinued treatment due to these adverse events (compared with 0.9% of men in the placebo group) with resolution occurring after discontinuation of drug. This medicine was then recommended for female pattern hair loss, as well.
Although finasteride works well to stop or reduce hair fall in a majority of people, it does not work as well at hair regrowth. Regular usage of finasteride leads to compliance issues & most people do not use finasteride continuously, because of the side effects it has on sexuality.
As the QR678® Neo has no real side effects & cause hair growth, especially in minoxidil & finasteride resistant cases, it is an interesting alternative to finasteride & is preferred by a large number of patients.
Clinical Trial Data
QR678® Neo has been through a Phase IV open label, prospective, single arm Multi-Ethnicity Clinical Trial, in which 2428 patients with androgenetic alopecia were administered the QR678 Neo formulation into the scalp skin.
The treatment regimen comprised 8 sessions, 3 weeks apart, till 8 sessions were completed. Hair pull test, video-microscopic & global images were taken at baseline, 4th session, and 8th session. Relevant safety assessments through physical examination, questionnaires & appropriate laboratory examination were conducted throughout the study.
The treatment was effective in improving the appearance of scalp hair & slowing the loss of hair in men & women with patterned hair loss. Improvement in hair growth with therapy was evident as early as after 4 sessions for all measured endpoints. At one year, a statistically significant increase in total hair count (P=0.002) continued to be seen. After 8 sessions, global photographs showed improvement from baseline for 91% patients, with an average score of 8 at the end of the 8th session.
The findings of this study suggest that the beneficial clinical effects of this therapy are similar in men & women, across different age groups, & in patients irrespective of the presence of metabolic disorders like diabetes, hypertension, hypercholesterolemia etc. There was a negative correlation between the duration & stage of hair loss, & the degree of improvement.
Synergistic use of QR678® Neo minoxidil & finasteride in
Female Pattern Alopecia:
In patients with advanced hair fall & advanced alopecia, we have used the QR 678® in combination with topical minoxidil lotion & oral finasteride tablets and seen additive & synergistic effects.
Hair transplant in Female Pattern Alopecia & effects of QR 678®
Hair transplant therapy like Follicular Unit Extraction (FUE) & Follicular Unit Transplantation (FUT) are used in advanced alopecia. But, hair transplants are invasive. The market for hair transplantation is dwarfed by the immense market for products that treat hair loss without surgery.
The major challenge for hair transplant is that only the occipital scalp hair (hair at back of scalp) is Dihydrotestosterone (DHT) resistant. This is the area from where donor hair is taken for the balding areas, in a hair transplant surgery. The problems with this approach are mainly two-fold:
A limited amount of donor hair The continued falling of the hair located at the frontal & vertex areas of the scalp, as the androgenetic alopecia progresses. Resultantly, the hair transplants need repetition & supportive medical therapy (minoxidil & finasteride) to maintain the initial results.
While the QR 678® can assist to reduce the chances of hair transplantation in early alopecia by arresting hair fall & causing hair growth, the QR 678® also works well to support the existing hair & the transplanted hair post hair transplant surgery.
Best treatment for Androgenetic Alopecia
Combination therapy using the QR 678® hair growth therapy, topical minoxidil, oral finasteride & hair transplant surgery where required may give the best results for androgenetic alopecia & male pattern baldness.
QR678 NEO: NATURAL INGREDIENTS
BACKED BY SCIENCE
The QR678® Neo therapy is a proprietary, first-in-class therapy, backed by science that arrests hair fall & increases the thickness, number, and density of existing hair follicles leading to greater coverage in hair loss. The biomimetic polypeptides used in the formulation mimic the action of specific human hair growth related growth factors that are naturally present in our scalp, making it natural growth factor-based treatment. The polypeptides penetrate deep into the scalp and provide nourishment to the scalp, which results in hair growth. Unlike other treatments that contains minoxidil & finasteride, QR678® Neo therapy is completely devoid of side effects.
HOW DOES IT WORK?
MEDIA INTERVIEWS
TESTIMONIALS
OUR PARTNERS

















FAQ'S
How is QR678® Neo better than other hair fall treatments?
QR678® Neo is a mixture of natural growth factors that are already present in the scalp and therefore it is completely safe to use on everybody. It has the right concentration of specific hair growth factors and QR678® Neo does not have any side effects, is performed as an outpatient procedure and the results are visible within eight weeks of starting the treatment. You can listen for yourself what the patients say about QR678® Neo hair fall treatment https://www.youtube.com/watch?v=pIvjpQgSsrc
How often do I need to repeat QR678® Neo treatment?
QR678® Neo injections are administered in 8-12 sessions in about 3-4 weeks apart. The results are visible after few weeks. Once you have achieved the desired results the injections need not be repeated unless the hair fall starts again. This is because QR678® Neo make the hair follicles healthy. Get to know more about why QR678® Neo is highly effective in treating hair fall and increasing hair growth. http://www.qr678.in/
What precautions should I take after QR678® Neo treatment?
QR678® Neo is a completely safe treatment without side effects and you can go about your daily routine after the sessions. The only care you need to take is to use a gentle shampoo not before the next day of the treatment and avoid scratching and rubbing the treating area. If you have any other problem then consult your dermatologist. https://www.youtube.com/watch?v=LNm2z3EGLxg
Can QR678® Neo be used after hair transplant?
Yes, using QR678® Neo after hair transplant produces very successful results. QR678® Neo gives faster density and reduces the catagen loss of transplanted hair. The growth factors in QR678® Neo help in faster healing and activate the dormant follicles and thus - Increase the viability of hair follicles - Improve healing and repairing of tissue process - Strengthen the inactive hair follicles and stimulate hair growth - Improves the health of existing hair and they will become fuller and stronger QR678® Neo can be given in multiple treatment sessions after three to four weeks after successful hair transplant surgery. QR678® Neo can also be used before the hair transplant surgery to reduce the area of transplant and improve the results.
How is QR678® Neo better than PRP and stem cell hair fall treatment?
QR678® Neo is a relatively new treatment for hair fall and hair regrowth but in the short time of its invention QR678® Neo has proven to be more effective than PRP and stem cell treatment for reducing hair fall. QR678® Neo produces longer lasting and better results and is completely safe and without any side effects. QR678® Neo can help treat and rogenetic alopecia, female pattern hair loss, and hair loss because of chemotherapy, seborrheic dermatitis, and alopecia areata. PRP and stem cell on the other hand do not have the specific concentration of required growth factors, they lack standardisation in terms of treatment protocols and preparation of the solution injected and lack good quality studies to prove their benefits. produce guaranteed results and can cause tightness and tenderness in scalp, scar tissue formation, calcification of the injected points, and formation of scar tissues. Both processes are still under investigation and their results are still awaiting confirmation. Dr Rinky Kapoor of The Esthetic Clinics explains the difference between QR678® Neo and stem cell here: https://www.youtube.com/watch?v=wgNihm8ciu4 and https://www.youtube.com/watch?v=ZUWXKzVh0PM
Can genetic hair loss be treated?
Yes genetic hair loss can be treated if the treatment is started in time. Heredity hair loss usually runs in the family and can affect both men and women. If you too have a close elder relative who lost his or hair in a patterned baldness then you should keep a close eye on your hair too. Hereditary hair loss usually happens in a pattern; M or W shaped in men and along the hair part in women. See the doctor immediately if you suffer from hair loss. There are many common and successful treatments available for genetic hair loss which include
- Topical and oral medications: These include Minoxidil topical lotion and Finasteride tablets.
- Hair transplantation using FUT and FUE methods.
- Nutritional supplements: Your physician might also suggest some hair supplements that can help arrest hair fall. Dietary supplements containing Vitamin A, C, and Biotin, zinc, and iron can also help control hair fall caused because of genetics.
Treatment option for Chemotherapy induced Alopecia?
Alopecia post chemotherapy is a very common consequence of cancer treatments and therapies and it is often the most psychologically and socially devastating side effect. Many patients shy away from the treatments because of fear of hair fall and hair loss. However, in most cases, such alopecia is reversible but the recovery takes several months and even up to a year. Some basic latest treatment methods will speed up the healing: - Scalp Cooling: This is a FDA approved method for patients suffering from breast cancer. Scalp cooling or hypothermia results in constriction of the blood vessels in the scalp, which in turn leads to reduced delivery of chemotherapy to the hair. Scalp cooling devices consist of cooling unit that circulate the coolant on the scalp. This is done about 30 minutes before the start of chemotherapy. Patients can feel some mild discomfort, nausea, and dry skin because of scalp cooling. - Topical Minoxidil: Minoxidil helps by increasing the blood flow to the hair follicle thereby preventing miniaturization of hair follicle. - QR678® Neo hair regrowth treatment: QR678® Neo is a FDA approved, completely safe hair regrowth treatment that has no side effects or causes any discomfort to the patient. Clinical trials have proved QR678 to be effective in treatment of chemotherapy induced alopecia in both men and women. The results of QR678® Neo last lifelong.
FOR MORE QUERIES
QR678 NEO: NATURAL INGREDIENTS
BACKED BY SCIENCE
The QR678® Neo therapy is a proprietary, first-in-class therapy, backed by science that arrests hair fall & increases the thickness, number, and density of existing hair follicles leading to greater coverage in hair loss. The biomimetic polypeptides used in the formulation mimic the action of specific human hair growth related growth factors that are naturally present in our scalp, making it natural growth factor-based treatment. The polypeptides penetrate deep into the scalp and provide nourishment to the scalp, which results in hair growth. Unlike other treatments that contains minoxidil & finasteride, QR678® Neo therapy is completely devoid of side effects.
HOW DOES IT WORK?

QR678® Neo ADVANTAGES

(Derived From Plants)

Quick & Effective (Single
Session in 10 Minutes)

Non Surgical

Success Rate
13+ Published Researched Clinical Trials Demonstrating Efficacy
LIFE CHANGING RESULTS
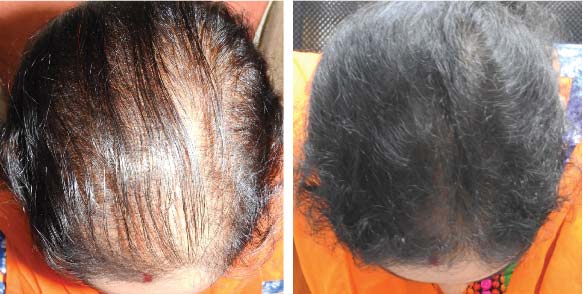
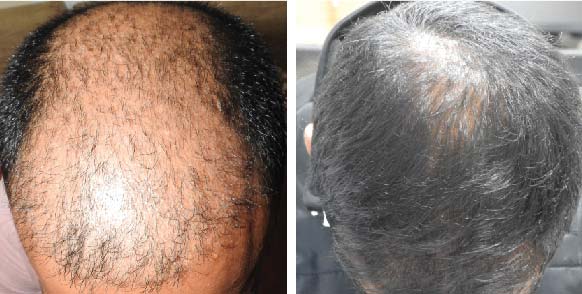
PRE TREATMENT
POST 6 SESSIONS
PRE TREATMENT
POST 6 SESSIONS

PRE TREATMENT
POST 6 SESSIONS
25 year old female from alopecia areata, was also diagnosed with hypothyroid. She achieved impressive results with QR678, a completely steroid-off treatment.

PRE TREATMENT
POST 6 SESSIONS
39 year old male suffering from male pattern hair loss for 15 years gained a significant amount of hair with QR678
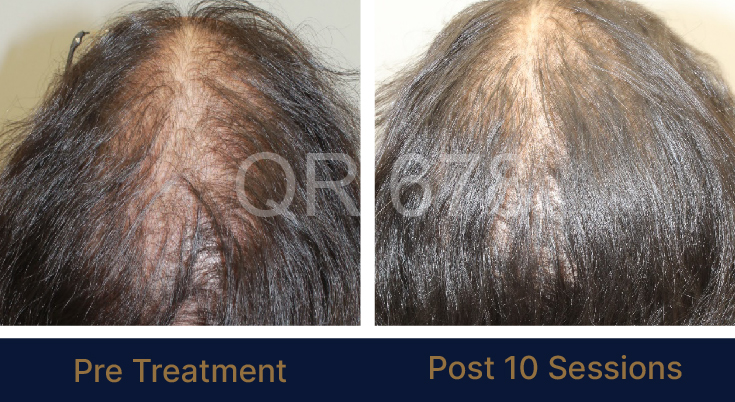
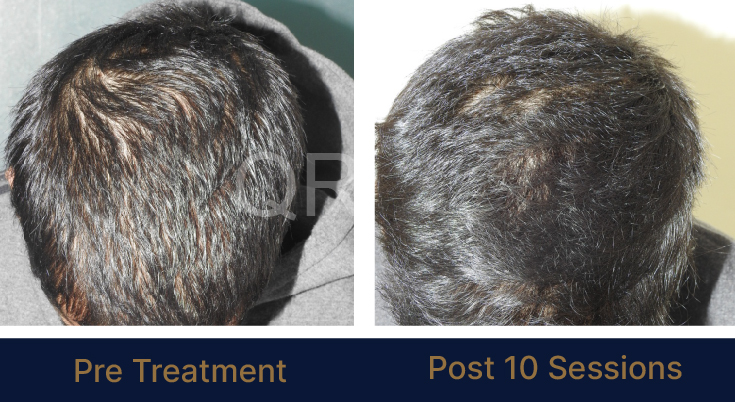
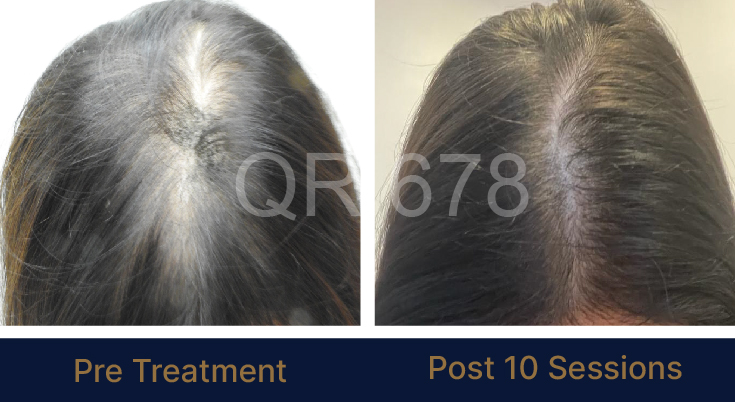
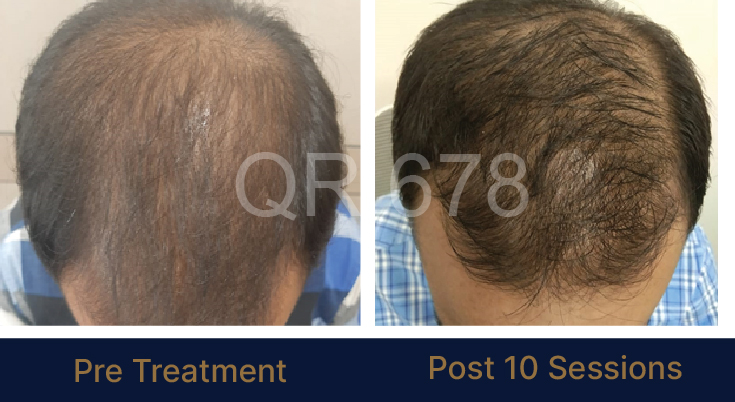
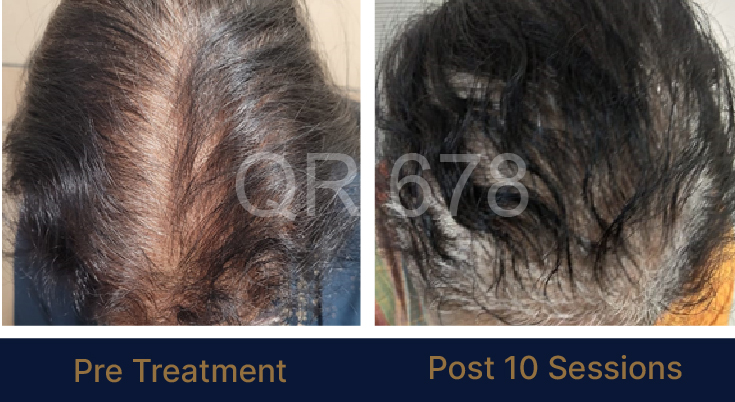
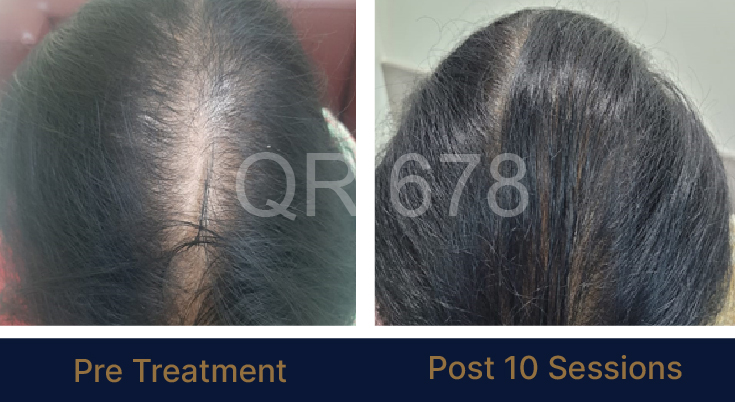
MEDIA INTERVIEWS
TESTIMONIALS
HIGHLIGHTS IN MEDICAL CONFERENCES





CUTICON – December 2023









GLOBAL PRESENCE
OUR PARTNERS

















FAQ'S
How is QR678® Neo better than other hair fall treatments?
QR678® Neo is a mixture of natural growth factors that are already present in the scalp and therefore it is completely safe to use on everybody. It has the right concentration of specific hair growth factors and QR678® Neo does not have any side effects, is performed as an outpatient procedure and the results are visible within eight weeks of starting the treatment. You can listen for yourself what the patients say about QR678® Neo hair fall treatment https://www.youtube.com/watch?v=pIvjpQgSsrc
How often do I need to repeat QR678® Neo treatment?
QR678® Neo injections are administered in 8-12 sessions in about 3-4 weeks apart. The results are visible after few weeks. Once you have achieved the desired results the injections need not be repeated unless the hair fall starts again. This is because QR678® Neo make the hair follicles healthy. Get to know more about why QR678® Neo is highly effective in treating hair fall and increasing hair growth. http://www.qr678.in/
What precautions should I take after QR678® Neo treatment?
QR678® Neo is a completely safe treatment without side effects and you can go about your daily routine after the sessions. The only care you need to take is to use a gentle shampoo not before the next day of the treatment and avoid scratching and rubbing the treating area. If you have any other problem then consult your dermatologist. https://www.youtube.com/watch?v=LNm2z3EGLxg
Can QR678® Neo be used after hair transplant?
Yes, using QR678® Neo after hair transplant produces very successful results. QR678® Neo gives faster density and reduces the catagen loss of transplanted hair. The growth factors in QR678® Neo help in faster healing and activate the dormant follicles and thus - Increase the viability of hair follicles - Improve healing and repairing of tissue process - Strengthen the inactive hair follicles and stimulate hair growth - Improves the health of existing hair and they will become fuller and stronger QR678® Neo can be given in multiple treatment sessions after three to four weeks after successful hair transplant surgery. QR678® Neo can also be used before the hair transplant surgery to reduce the area of transplant and improve the results.
How is QR678® Neo better than PRP and stem cell hair fall treatment?
QR678® Neo is a relatively new treatment for hair fall and hair regrowth but in the short time of its invention QR678® Neo has proven to be more effective than PRP and stem cell treatment for reducing hair fall. QR678® Neo produces longer lasting and better results and is completely safe and without any side effects. QR678® Neo can help treat and rogenetic alopecia, female pattern hair loss, and hair loss because of chemotherapy, seborrheic dermatitis, and alopecia areata. PRP and stem cell on the other hand do not have the specific concentration of required growth factors, they lack standardisation in terms of treatment protocols and preparation of the solution injected and lack good quality studies to prove their benefits. produce guaranteed results and can cause tightness and tenderness in scalp, scar tissue formation, calcification of the injected points, and formation of scar tissues. Both processes are still under investigation and their results are still awaiting confirmation. Dr Rinky Kapoor of The Esthetic Clinics explains the difference between QR678® Neo and stem cell here: https://www.youtube.com/watch?v=wgNihm8ciu4 and https://www.youtube.com/watch?v=ZUWXKzVh0PM
Can genetic hair loss be treated?
Yes genetic hair loss can be treated if the treatment is started in time. Heredity hair loss usually runs in the family and can affect both men and women. If you too have a close elder relative who lost his or hair in a patterned baldness then you should keep a close eye on your hair too. Hereditary hair loss usually happens in a pattern; M or W shaped in men and along the hair part in women. See the doctor immediately if you suffer from hair loss. There are many common and successful treatments available for genetic hair loss which include
- Topical and oral medications: These include Minoxidil topical lotion and Finasteride tablets.
- Hair transplantation using FUT and FUE methods.
- Nutritional supplements: Your physician might also suggest some hair supplements that can help arrest hair fall. Dietary supplements containing Vitamin A, C, and Biotin, zinc, and iron can also help control hair fall caused because of genetics.
Treatment option for Chemotherapy induced Alopecia?
Alopecia post chemotherapy is a very common consequence of cancer treatments and therapies and it is often the most psychologically and socially devastating side effect. Many patients shy away from the treatments because of fear of hair fall and hair loss. However, in most cases, such alopecia is reversible but the recovery takes several months and even up to a year. Some basic latest treatment methods will speed up the healing: - Scalp Cooling: This is a FDA approved method for patients suffering from breast cancer. Scalp cooling or hypothermia results in constriction of the blood vessels in the scalp, which in turn leads to reduced delivery of chemotherapy to the hair. Scalp cooling devices consist of cooling unit that circulate the coolant on the scalp. This is done about 30 minutes before the start of chemotherapy. Patients can feel some mild discomfort, nausea, and dry skin because of scalp cooling. - Topical Minoxidil: Minoxidil helps by increasing the blood flow to the hair follicle thereby preventing miniaturization of hair follicle. - QR678® Neo hair regrowth treatment: QR678® Neo is a FDA approved, completely safe hair regrowth treatment that has no side effects or causes any discomfort to the patient. Clinical trials have proved QR678 to be effective in treatment of chemotherapy induced alopecia in both men and women. The results of QR678® Neo last lifelong.
FOR MORE QUERIES
Country: India Bangladesh Kuwait Thailand UAE