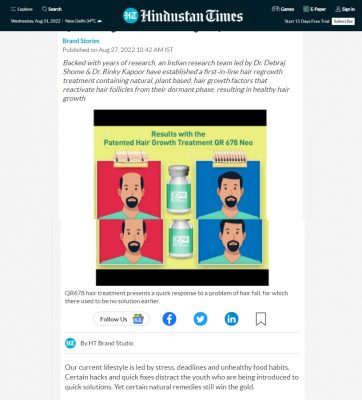
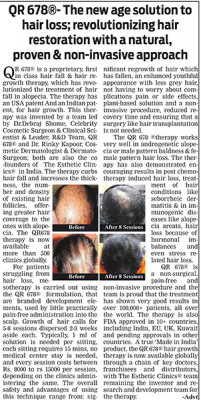
Evaluation of the safety and effectiveness of intradermal administration of QR678® anti-hair loss formulation - A phase-IV, open-label, single-arm multi-ethnicity clinical trial
Research Overview
Clinical documentation of hair thinning and increased hair shedding remains an important component of dermatology practice, particularly in patients presenting with patterned hair changes and other reasons for hair thinning encountered in routine clinics. Evaluation commonly involves serial assessment of scalp condition, follicular appearance, and changes observed across successive visits, interpreted within established hair cycle phases and known variability in hair biology.
This multicentric Phase IV study documents hair and scalp parameters observed following intradermal scalp application of a proprietary formulation, QR678, in adults presenting with patterned hair thinning. The investigation focuses on structured clinical assessments performed under routine practice conditions, contributing observational data related to hair and scalp characteristics and the broader scalp environment recorded over the study period.
Study Design and Clinical Framework
The study was conducted as a multicentric, open-label Phase IV clinical investigation across dermatology clinics in multiple geographic regions. Adult participants between 18 and 65 years of age presenting with patterned hair thinning were enrolled based on predefined inclusion criteria.
The protocol involved intradermal scalp application administered at fixed intervals under clinical supervision. The study did not include randomization, placebo control, or comparator arms. While the original trial design was interventional, the documentation presented here is limited to clinical observation and does not infer therapeutic outcomes.
Hair and Scalp Assessment Methods
Participants underwent structured evaluations at baseline and at scheduled follow-up visits. Assessment tools included clinical scalp examination, hair pull testing as a clinical indicator used in differentiating hair shedding vs hair fall, global photographic documentation under standardized conditions, and videomicroscopic evaluation of hair and scalp parameters.
Participant-reported feedback related to procedural experience and scalp sensations was recorded using standardized questionnaires. All assessment methods reflect commonly employed dermatological documentation techniques rather than efficacy-driven outcome measures.
Observational Findings and Hair Cycle Considerations
Clinical observations were interpreted within established frameworks of hair biology and hair cycle phases. Documentation focused on changes noted during repeated assessments, without assigning causality, permanence, or predictive value to the findings.
The study did not attempt to categorize observations under disease-specific mechanisms or consumer-oriented distinctions beyond descriptive recording. All findings were documented in alignment with routine dermatological evaluation practices.
Tolerability and Safety Observations
Tolerability was monitored throughout the study period. Observations were primarily localized and transient, including mild procedural discomfort or temporary scalp sensitivity associated with intradermal application.
No systemic adverse events were documented during the study timeframe. Safety-related observations were recorded descriptively and without extrapolation beyond the duration of clinical follow-up.
Scope, Limitations, and Research Relevance
As a Phase IV clinical investigation without control groups or blinding, the study is subject to inherent methodological limitations. It does not establish causal relationships, comparative effectiveness, or long-term outcomes.
Within these constraints, the study contributes large-scale clinical documentation of hair and scalp parameters observed across diverse patient populations under routine dermatological use. The findings serve as reference material for clinicians and researchers and may inform the design of future controlled studies focused on scalp and follicular assessment.
Disclaimer:
The research papers and clinical articles referenced on this website are peer-reviewed scientific publications authored by qualified medical professionals and represent the observations and conclusions of the respective authors, based on their individual clinical research.
These clinical references are provided for informational and educational purposes and should not be interpreted as promotional claims or outcome guarantees by QR678®. Consumers are advised to consult a qualified healthcare professional for medical interpretation or hair- or scalp-related concerns.
QR678®: SCIENCE Backed Research & Development Proprietary Product Technology
The QR678® research & development platform technology is a proprietary first-in-class technology. This science backed technology is giving rise to multiple products that arrest hair fall and address the thickness, density of hair follicles leading to greater coverage in hair loss. This R&D technology deals with multiple human hair growth related growth factors that are naturally present in our scalp making products derived from these completely natural. Research on this platform technology has resulted in this being awarded USA & Indian patents and has led to over 16+ clinical research papers being published with products derived from this unique technology. The hair growth factors penetrate deep into the scalp and provide nourishment to the scale. Unlike other treatments that contains harmful medication, products derived from QR678® are almost completely devoid of side effects.
HOW DOES IT WORK?
QR678® ADVANTAGES

(Derived From Plants)

Quick & Effective (Single
Session in 10 Minutes)


Non Surgical

Success Rate
13+ Published Researched Clinical Trials Demonstrating Efficacy
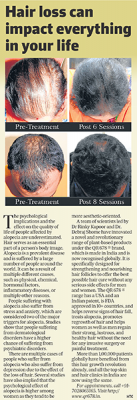
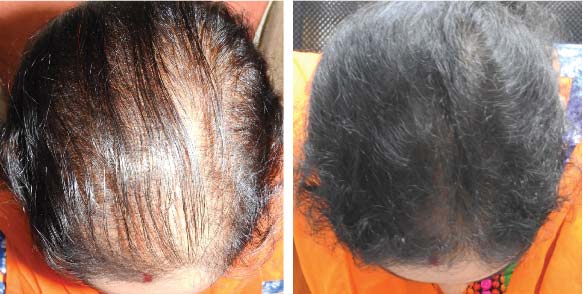
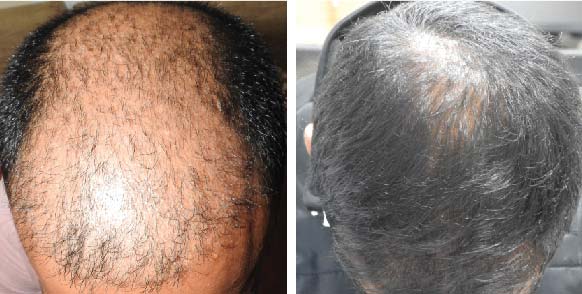
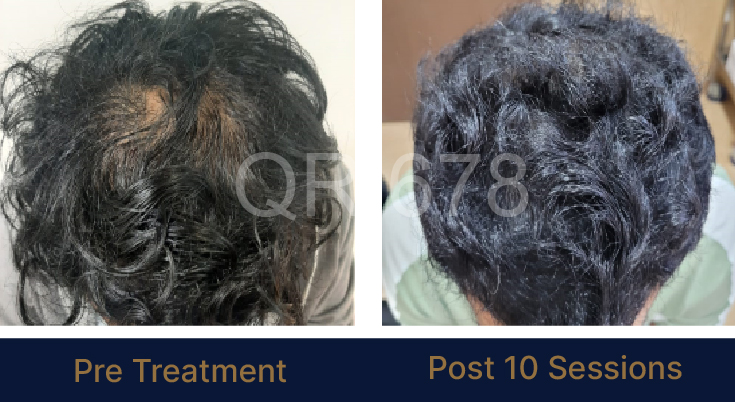
LIFE CHANGING RESULTS
PRE TREATMENT
POST 6 SESSIONS
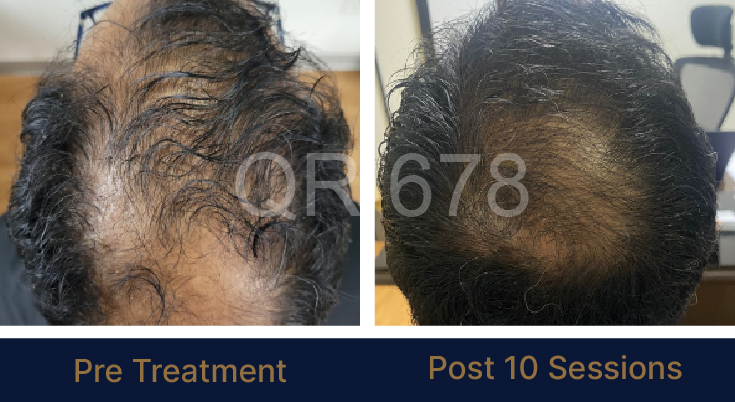
PRE TREATMENT
POST 6 SESSIONS

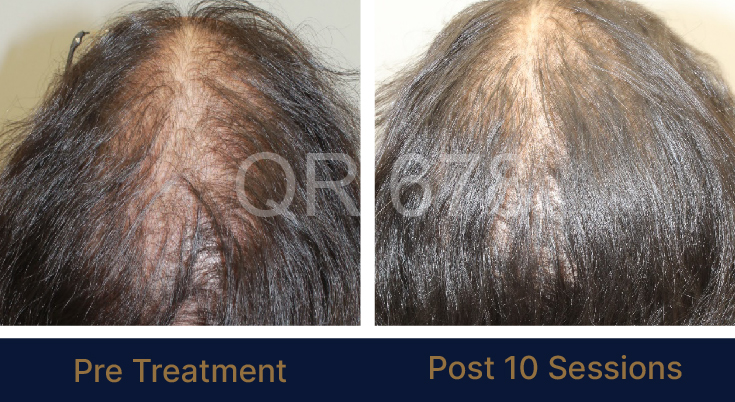
PRE TREATMENT
POST 6 SESSIONS
25 year old female from alopecia areata, was also diagnosed with hypothyroid. She achieved impressive results with QR678, a completely steroid-off treatment.

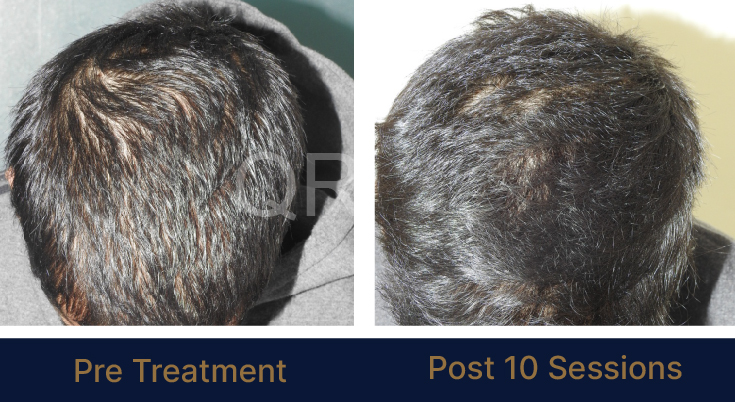
PRE TREATMENT
POST 6 SESSIONS
39 year old male suffering from male pattern hair loss for 15 years gained a significant amount of hair with QR678
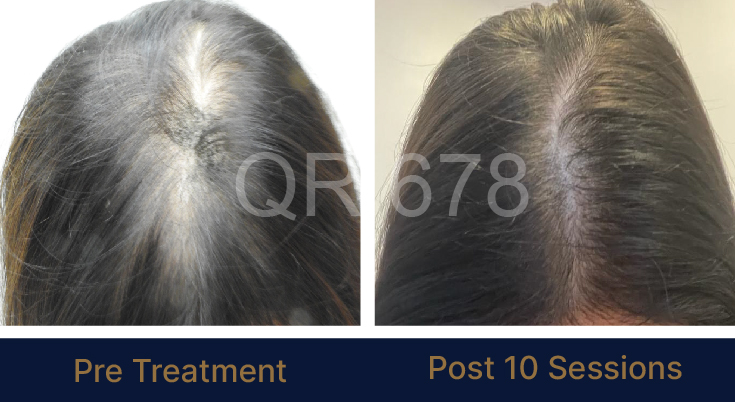
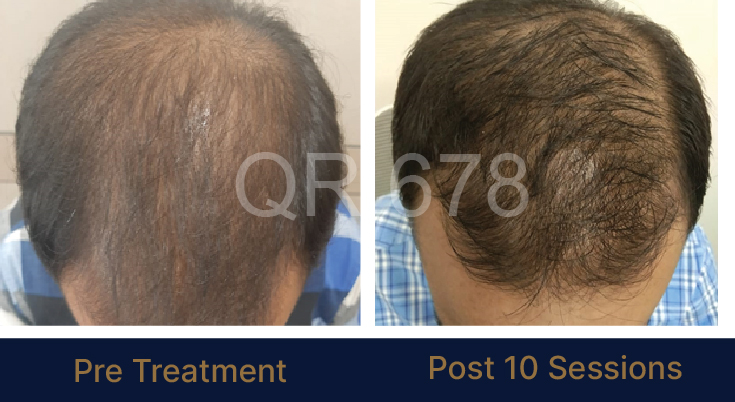
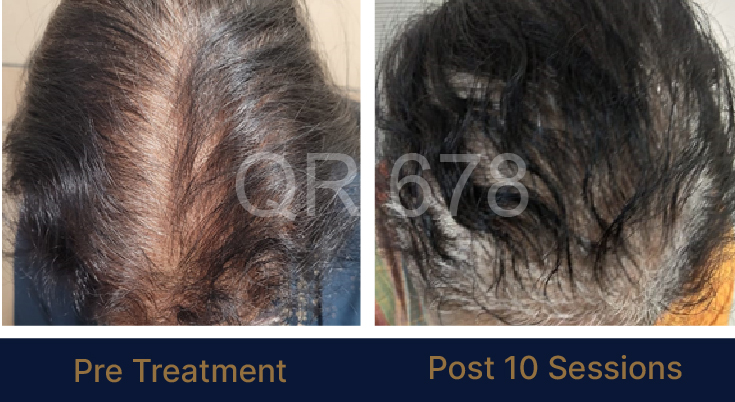
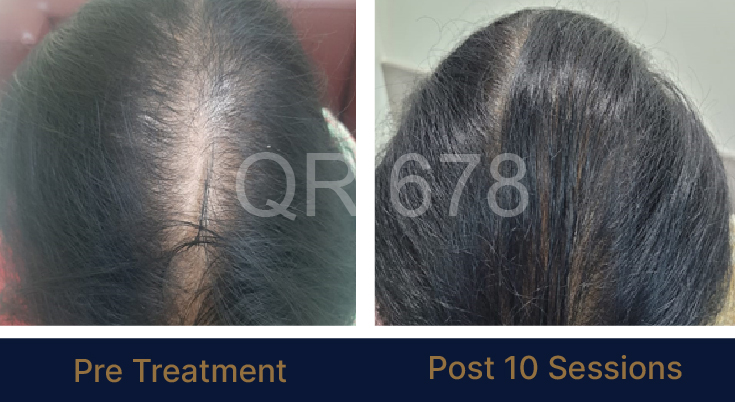
MEDIA INTERVIEWS
TESTIMONIALS
HIGHLIGHTS IN MEDICAL CONFERENCES





CUTICON – December 2023
Dazzling moments at Cuticon 2023, Gujarat! QR678 took centre stage, earning accolades from top medical professionals.





QR678 made it’s presence felt at FADS (Face Aesthetics Dermatologists Society), the 7th International Conference held at Goa. Renowned dermatologists got QR678 Neo administered and shared their positive experience.




QR678® drew attention with an enlightening session and live demonstration at CDCON 2022 – a platform to challenge changing practices along the lines of Exploration and Innovation.
GLOBAL PRESENCE

OUR PARTNERS

















FAQ'S
How is QR678® better than other hair fall treatments?
QR678® is a mixture of natural growth factors that are already present in the scalp and therefore it is completely safe to use on everybody. It has the right concentration of specific hair growth factors and QR678® does not have any side effects, is performed as an outpatient procedure and the results are visible within eight weeks of starting the treatment. You can listen for yourself what the patients say about QR678® hair fall treatment https://www.youtube.com/watch?v=pIvjpQgSsrc
How often do I need to repeat QR678® treatment?
QR678® injections are administered in 8-12 sessions in about 3-4 weeks apart. The results are visible after few weeks. Once you have achieved the desired results the injections need not be repeated unless the hair fall starts again. This is because QR678® make the hair follicles healthy. Get to know more about why QR678® is highly effective in treating hair fall and increasing hair growth. http://www.qr678.in/
What precautions should I take after QR678® treatment?
QR678® is a completely safe treatment without side effects and you can go about your daily routine after the sessions. The only care you need to take is to use a gentle shampoo not before the next day of the treatment and avoid scratching and rubbing the treating area. If you have any other problem then consult your dermatologist. https://www.youtube.com/watch?v=LNm2z3EGLxg
Can QR678® be used after hair transplant?
Yes, using QR678® after hair transplant produces very successful results. QR678® gives faster density and reduces the catagen loss of transplanted hair. The growth factors in QR678® Neo help in faster healing and activate the dormant follicles and thus
- Increase the viability of hair follicles
- Improve healing and repairing of tissue process
- Strengthen the inactive hair follicles and stimulate hair growth
- Improves the health of existing hair and they will become fuller and stronger
QR678® can be given in multiple treatment sessions after three to four weeks after successful hair transplant surgery. QR678® can also be used before the hair transplant surgery to reduce the area of transplant and improve the results.
How is QR678® better than PRP and stem cell hair fall treatment?
QR678® is a relatively new treatment for hair fall and hair regrowth but in the short time of its invention QR678® has proven to be more effective than PRP and stem cell treatment for reducing hair fall. QR678® produces longer lasting and better results and is completely safe and without any side effects. QR678® can help treat and rogenetic alopecia, female pattern hair loss, and hair loss because of chemotherapy, seborrheic dermatitis, and alopecia areata.
PRP and stem cell on the other hand do not have the specific concentration of required growth factors, they lack standardisation in terms of treatment protocols and preparation of the solution injected and lack good quality studies to prove their benefits. produce guaranteed results and can cause tightness and tenderness in scalp,
scar tissue formation, calcification of the injected points, and formation of scar tissues. Both processes are still under investigation and their results are still awaiting confirmation. Dr Rinky Kapoor of The Esthetic Clinics explains the difference between QR678® and stem cell here: https://www.youtube.com/watch?v=wgNihm8ciu4 and
Can genetic hair loss be treated?
Yes genetic hair loss can be treated if the treatment is started in time. Heredity hair loss usually runs in the family and can affect both men and women. If you too have a close elder relative who lost his or hair in a patterned baldness then you should keep a close eye on your hair too. Hereditary hair loss usually happens in a pattern; M or W shaped in men and along the hair part in women. See the doctor immediately if you suffer from hair loss. There are many common and successful treatments available for genetic hair loss which include
- Topical and oral medications: These include Minoxidil topical lotion and Finasteride tablets.
- Hair transplantation using FUT and FUE methods.
- Nutritional supplements: Your physician might also suggest some hair supplements that can help arrest hair fall. Dietary supplements containing Vitamin A, C, and Biotin, zinc, and iron can also help control hair fall caused because of genetics.
Treatment option for Chemotherapy induced Alopecia?
Alopecia post chemotherapy is a very common consequence of cancer treatments and therapies and it is often the most psychologically and socially devastating side effect. Many patients shy away from the treatments because of fear of hair fall and hair loss. However, in most cases, such alopecia is reversible but the recovery takes several months and even up to a year. Some basic latest treatment methods will speed up the healing:
- Scalp Cooling: This is a FDA approved method for patients suffering from breast cancer. Scalp cooling or hypothermia results in constriction of the blood vessels in the scalp, which in turn leads to reduced delivery of chemotherapy to the hair. Scalp cooling devices consist of cooling unit that circulate the coolant on the scalp. This is done about 30 minutes before the start of chemotherapy. Patients can feel some mild discomfort, nausea, and dry skin because of scalp cooling.
- Topical Minoxidil: Minoxidil helps by increasing the blood flow to the hair follicle thereby preventing miniaturization of hair follicle.
- QR678® hair regrowth treatment: QR678® is a FDA approved, completely safe hair regrowth treatment that has no side effects or causes any discomfort to the patient. Clinical trials have proved QR678 to be effective in treatment of chemotherapy induced alopecia in both men and women. The results of QR678® last lifelong.
FOR MORE QUERIES
QR678® 2019-26. Owned by Esthetic Products International LLP, Sofia, Bulgaria.
The QR678® research & development product platform technology is developing a group of patented, proprietary products. Any infringement of any kind will invite legal action.
Geographies Addressed: Bulgaria, European Union, Kuwait, Malaysia, Qatar, Oman, Serbia, India
Registration process underway: Canada, Phillipines, UAE, South Africa, Uganda, Indonesia, Singapore, Srilanka, Maldives, Bangladesh, Pakistan, Nepal
Disclaimer: QR678® is a proprietary research and technology platform focused on scientific innovation in hair and scalp science.
This website is intended solely for the dissemination of scientific, educational, and informational content relating to research and technology development. It does not offer products for sale, nor does it provide medical or therapeutic advice.